Insights+: The US FDA New Drug Approvals in September 2023
Shots:
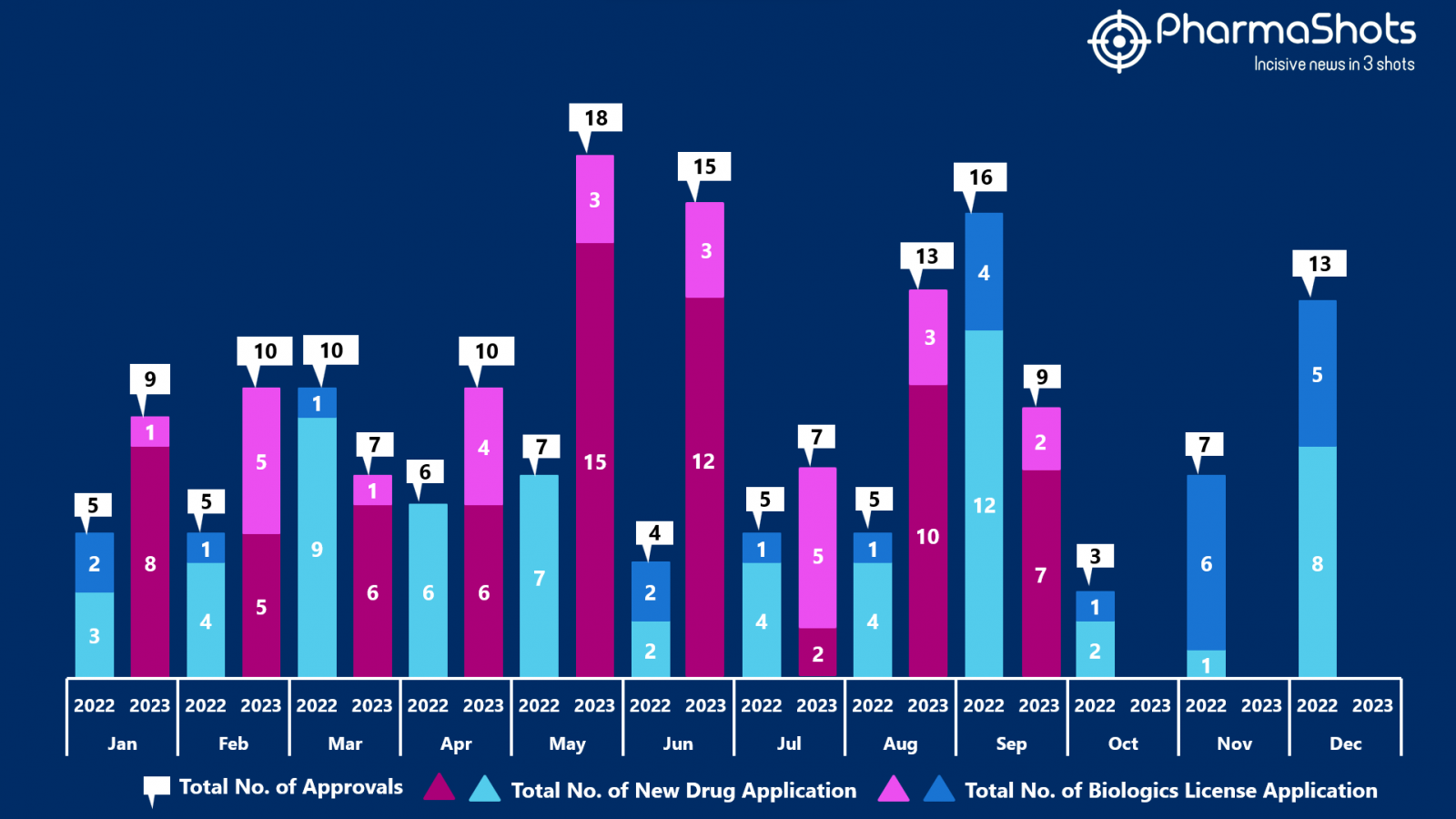
- The US FDA approved 7 NDAs and 2 BLAs in September 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 98 novel products in 2023
- In September 2023, the major highlights drugs were Ojjaara (momelotinib) approval for myelofibrosis patients with anemia, and Entyvio (vedolizumab) for subcutaneous administration to treat active ulcerative colitis
- PharmaShots has compiled a list of a total of 9 new drugs approved by the US FDA in September 2023
1. BioLineRx’s Aphexda (motixafortide) + Filgrastim Receives the US FDA’s Approval for Multiple Myeloma
Aphexda and Filgrastim
Active ingredient: motixafortide Approved: Sept 12, 2023
Company: BioLineRx Disease: Multiple Myeloma
- The US FDA has approved motixafortide + filgrastim (G-CSF) to mobilize hematopoietic stem cells (HSC) to the peripheral blood for collection & subsequent autologous transplantation in patients with MM. The product is expected to be available in Sept 2023
- The approval was based on the 2-part P-III study (GENSIS) evaluating motixafortide + filgrastim vs PBO + filgrastim. The first part of the study focused on determining dosage and incl. 12 patients while the second part randomly assigned 122 patients in a ratio (2:1)
- The study met its 1EPs i.e., 67.5% vs 9.5% of patients reached the stem cell collection goal of 6×10^6 CD34+ cells/kg within 2 apheresis sessions as measured by the central laboratory; 92.5% vs 21.4% by local laboratories. The safety was evaluated in 92 patients with Aphexda (1.25mg/kg, SC) + filgrastim and 42 with PBO + filgrastim
2. GSK Receives the US FDA’s Approval of Ojjaara (momelotinib) for Myelofibrosis Patients with Anemia
Ojjaara
Active ingredient: momelotinib Approved: Sept 18, 2023
Company: GSK Disease: Myelofibrosis
- The US FDA has approved Ojjaara for intermediate or high-risk myelofibrosis, incl. primary myelofibrosis or secondary myelofibrosis (post-polycythemia vera & post-essential thrombocythemia) in adults with anemia
- The approval was based on the P-III trial (MOMENTUM) & data for a subpopulation of adult patients with anemia from the P-III (SIMPLIFY-1) trial evaluating momelotinib. The (MOMENTUM) trial met all 1EPs & 2EPs and demonstrated significant improvements in myelofibrosis-associated symptoms, anemia measures, and spleen response with favorable safety over danazol
- In (SIMPLIFY-1), momelotinib showed noninferiority to ruxolitinib in spleen volume response at a reduction of ≥35% as well as improvement in transfusion dependence rates
Jardiance
Active ingredient: empagliflozin Approved: Sept 25, 2023
Company: Boehringer Ingelheim and Eli Lilly Disease: Chronic Kidney Disease
- The US FDA has approved Jardiance (10mg) to reduce the risk of sustained decline in eGFR, end-stage kidney disease, CV death & hospitalization in adults with CKD at risk of progression
- The approval was based on the P-III trial (EMPA-KIDNEY) evaluating Jardiance (10mg, qd) vs PBO in 6609 adults with CKD with/out type 2 diabetes across 8 countries, showed 28% relative risk reduction (absolute risk reduction 3.6% per patient-year at risk) over PBO when used with SoC for the composite 1EPs of kidney disease progression or cardiovascular death, event rate for Jardiance was 13.1% vs 16.9%
- The results were consistent across prespecified subgroups & demonstrated a 14% reduction in risk of first & recurrent hospitalization
Likmez
Active ingredient: metronidazole Approved: Sept 25, 2023
Company: Saptalis Pharmaceuticals Disease: Antimicrobial Infections
- The US FDA has approved liquid oral reformulation of the antibiotic metronidazole, ATI-1501 for the treatment of antimicrobial infections in patients with difficulty swallowing pills or who are unable to get injections. The product is expected to be available to patients shortly & the company bring additional products to market for infectious diseases
- The company expects to receive milestones and royalties from Saptalis in upcoming quarters based on the US FDA approval and Saptalis’ commercialization plans.
- Likmez oral suspension is supplied in a 200mL bottle containing 500mg/5mL of metronidazole in a strawberry peppermint flavor. Patent coverage provides drug market exclusivity through at least 2039
Entyvio
Active ingredient: vedolizumab Approved: Sept 27, 2023
Company: Takeda Disease: Ulcerative Colitis
- The US FDA has approved Entyvio (SC) for maintenance therapy in adults with moderately to severely active UC after induction therapy with Entyvio (IV). The therapy is expected to be available in the US as a single-dose pre-filled pen (ENTYVIO Pen) by the end of Oct
- The approval was based on the P-III study (VISIBLE 1) study evaluating Entyvio (SC) as maintenance therapy in 162 patients which showed that patients who received Entyvio SC (108mg, q2w) maintenance therapy achieved clinical remission (46% vs 14%) at 52wk. The safety profile was consistent with the known safety profile of Entyvio IV
- Vedolizumab IV received marketing authorization in 70+ countries, incl. the US & EU while the BLA for Entyvio (SC) is currently under the US FDA’s review for active Crohn’s disease
Ryzumvl
Active ingredient: phentolamine mesylate Approved: Sept 28, 2023
Company: Viatris and Ocuphire Pharma Disease: Pharmacologically-Induced Mydriasis
- The approval was based on the MIRA clinical program incl. P-IIb (MIRA-1), P-III (MIRA-2 & 3) studies in 553 patients aged 12-80yrs. & P-III (MIRA-4) pediatric trial evaluating Ryzumvi. Patients will receive either 2 drops of Ryzumvi or PBO in the study eye & 1 drop in the fellow eye, 1 hr. post pharmacologically-induced mydriasis
- In both trials, the percentage of patients with study eyes returning to ≤0.2 mm from baseline pupil diameter was greater at all time points measured from 60min. through 24hrs. Findings also showed that the change from maximum pupil dilation in study eyes and fellow eyes was different b/w the groups
- The product is expected to be available in the US in H1’24. The P-III trial (MIRA-4) showed that Ryzumvi rapidly reversed mydriasis
Exxua
Active ingredient: gepirone Approved: Sept 28, 2023
Company: Fabre-Kramer Pharmaceuticals Disease: Major Depressive Disorder
- The US FDA has approved Exxua (gepirone hydrochloride extended-release tablets) for adults with MDD. The therapy is expected to be available in pharmacies in early 2024 & is also being developed for other psychiatric disorders
- The efficacy of Exxua was based on two 8wk. PBO-controlled studies in a ratio (1:1) in adults aged 18-69yrs. The results showed that patients treated with Exxua (selective 5HT1a receptor agonist) achieved a greater improvement in the HAMD-17 total score at 8wk.
- Acc. to adverse event data collected in clinical trials, Exxua were found to be comparable to PBO & showed an overall acceptable safety profile with no significant adverse effect on weight, blood pressure, heart rate, or liver function
Pombiliti and Opfolda
Active ingredient: cipaglucosidase alfa-atga Approved: Sept 28, 2023
Company: Amicus Therapeutics Disease: Pompe Disease
- The US FDA has approved Pombiliti + Opfolda (65mg) for adults with LOPD who are not improving on their current enzyme replacement therapy. The approval was based on the P-III study (PROPEL) evaluating Pombiliti + Opfolda in ERT-experienced participants in a controlled setting
- Pombiliti + Opfolda is expected to be available in the US shortly & received BTD from the US FDA. The combination therapy was also approved for adults with LOPD in the EU & UK
- The company also provides a patient support program i.e., Amicus Assist which provides access to patients to use the treatment along with identifying possible sources of financial assistance
Rivfloza
Active ingredient: nedosiran Approved: Sept 28, 2023
Company: Novo Nordisk Disease: Primary Hyperoxaluria Type 1
- The US FDA has approved Rivfloza (80/128/160mg, qm) in children aged ≥9yrs. & adults with PH1 and relatively preserved kidney function. The approval was based on the P-II trial (PHYOX 2) trial evaluating Rivfloza vs PBO in 35 patients with PH1 or PH2 and an eGFR ≥30 mL/min/1.73 m2 along with interim data from P-III extension study (PHYOX 3)
- The (PHYOX 2) trial met its 1EPs & showed a reduction from baseline in 24hr.-urinary oxalate (Uox) excretion from Day 90-180, LS mean difference of AUC24-hour Uox was 4976 significant b/w both groups over 90 days
- In the (PHYOX 3) extension study, reductions in 24hr. Uox excretion was maintained in 13 patients who had received an additional 6mos. of treatment. Rivfloza is an RNAi therapy designed to lower urinary oxalate levels & is expected to be available in early 2024
Related Post: Insights+: The US FDA New Drug Approvals in August 2023





